mRNA Vaccines: A New Era in Preventive Medicine
VACCINES are one of the most powerful tools in modern science, essential for preventing infectious diseases worldwide. The history of vaccinology began in the early 1900s with the development of the first live attenuated vaccine against smallpox. Since then, this field has grown significantly, introducing various types of vaccines such as inactivated, subunit, toxoid, conjugate, DNA, viral-vectored, and more recently, mRNA-based vaccines.
Despite their success, traditional vaccines have struggled to address certain global health threats. Diseases like HIV, tuberculosis (TB), and malaria continue to cause millions of deaths each year, especially in low- and middle-income countries (LMICs). The only available vaccine for TB, known as BCG (Bacillus Calmette–Guérin), offers inconsistent protection and fails to prevent disease transmission. Meanwhile, no licensed vaccines exist yet for HIV or malaria. This has created an urgent need for new, adaptable vaccine platforms—leading to the rise of mRNA vaccine technology.
The success of mRNA vaccines during the COVID-19 pandemic opened the door for their broader application. These vaccines proved to be not only effective and safe, but also faster to develop and easier to produce at scale. As a result, there has been a rapid expansion of research into mRNA-based vaccines for a wide range of infectious diseases—including those for which vaccines have long remained elusive, like HIV, TB, and malaria. Furthermore, mRNA platforms are now being explored for cancer immunotherapies and treatments for genetic disorders through protein-replacement strategies.
Structure of mRNA
Messenger RNA (mRNA) is a single-stranded nucleic acid that carries genetic instructions from DNA to the cell's ribosomes, where proteins are made. In eukaryotic cells, mRNA is produced in the nucleus by RNA polymerase and then transported into the cytoplasm for translation. A mature mRNA molecule typically includes an open reading frame (ORF), which is the actual gene coding sequence, flanked by untranslated regions (UTRs) at the 5’ and 3’ ends. These UTRs play an essential role in regulating mRNA stability and translation.
At the 5’ end, eukaryotic mRNAs have a cap structure known as N7-methylguanosine. This cap is critical for initiating translation and helps protect the RNA from degradation. At the 3’ end, a long polyadenine tail (poly(A) tail) enhances mRNA stability and plays a role in efficient translation. Interestingly, recent studies suggest that shorter poly(A) tails may be associated with highly expressed genes, while longer tails may correspond to reduced translation efficiency. Another important feature is the 2’-O-methylation of the cap structure, which helps the immune system differentiate self mRNA from foreign or viral RNA, reducing unnecessary immune activation.
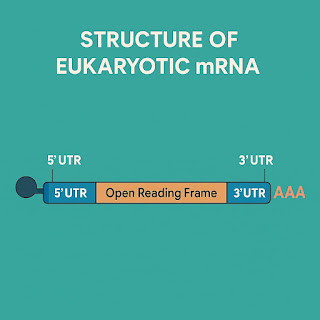 |
| Structure of mRNA |
Designing of mRNA Vaccines
The manufacturing of mRNA vaccines is a cell-free, in vitro process that involves:
1. Plasmid Design: A gene of interest is cloned downstream of a promoter in a plasmid.
2. Transformation & Selection: The plasmid is introduced into E. coli, grown in an antibiotic medium, and selected.
3. Template Preparation: The plasmid is purified and linearized using restriction enzymes.
4. In Vitro Transcription (IVT): Using RNA polymerase and NTPs, mRNA is transcribed from the linear DNA template.
5. Capping & Polyadenylation: The 5’ cap and 3’ poly(A) tail are added during or after transcription for stability and function.
 |
| Process of In-vitro mRNA transcription |
Increasing the Efficiency of mRNA Vaccines
Although mRNA vaccines are highly promising, they face certain challenges related to stability and immune recognition. To improve their effectiveness, several strategies have been developed. One common method is codon optimization, where rarely used codons in the gene sequence are replaced with ones more commonly used in human cells. This can enhance translation efficiency but must be done carefully, as excessive optimization may affect protein folding or function.
Another widely used technique is nucleoside modification, especially the substitution of uridine with pseudouridine or its derivatives. This modification helps the mRNA evade detection by innate immune receptors, thereby reducing inflammatory responses and side effects. Additionally, researchers often engineer the 5’ and 3’ UTRs to further increase mRNA stability and protein expression.
These optimizations collectively improve how well the mRNA functions inside the cell, enhancing its ability to generate a strong and effective immune response. However, it's important to ensure that the mRNA remains pure, as any impurities—such as leftover nucleotides or double-stranded RNA (dsRNA)—can reduce the efficiency of translation and trigger unwanted immune reactions.
Purification and Delivery of mRNA Vaccines
Purifying the mRNA after synthesis is a critical step in vaccine development. At the laboratory scale, common purification methods include DNase treatment, lithium chloride (LiCl) precipitation, cellulose-based chromatography, and various commercial purification kits. For larger-scale, GMP-compliant vaccine manufacturing, more advanced techniques are employed—such as ion-pair reverse-phase chromatography (IPC), ion-exchange chromatography (IEC), tangential flow filtration (TFF), and affinity chromatography. These techniques help remove contaminants like short abortive transcripts and dsRNA, ensuring a clean final product ready for clinical use.
Once purified, the next challenge is delivering the mRNA into human cells. Naked mRNA is fragile and easily degraded by enzymes (RNases) present in the body. Moreover, due to its negative charge, mRNA cannot easily pass through the cell membrane. To overcome this, lipid nanoparticles (LNPs) are used as delivery vehicles. These particles encapsulate the mRNA and protect it during transport, enabling it to enter cells efficiently. LNPs are composed of ionizable lipids, helper phospholipids, cholesterol, and PEG-lipids—each contributing to the stability, delivery, and uptake of the mRNA.
An emerging technology in this field is the use of self-amplifying mRNA (saRNA). This platform includes a viral replicase gene that allows the mRNA to replicate within the host cell, leading to higher antigen production with lower doses. This approach holds great promise for reducing manufacturing costs while maintaining strong immune responses, especially in settings where vaccine access is limited.
The development of mRNA vaccine technology marks a revolutionary advancement in the field of immunology and public health. Its advantages—rapid production, flexibility, safety, and high efficacy—make it ideal for responding to both new and persistent infectious threats. As research continues to evolve, there is strong hope that mRNA vaccines will soon provide protection against deadly diseases like HIV, tuberculosis, and malaria, which have long resisted traditional vaccine approaches.
Moreover, the possibility of multivalent mRNA vaccines—targeting multiple pathogens in a single shot—could transform disease prevention strategies across the world. With the progress we are witnessing today, we may soon experience a future where mRNA technology plays a key role in ending some of the world’s most challenging health crises.
Reference & Further Reading
Some of the scientific insights and information in this blog are adapted from a research article published in Frontiers in Immunology. For those interested in a deeper dive into the molecular details, vaccine development strategies, and clinical perspectives on mRNA vaccines, you can access the full article here:
🔗 Read the full article on Frontiers: https://doi.org/10.3389/fimmu.2023.1172691
(Title: "mRNA vaccines: a new opportunity for malaria, tuberculosis and HIV")
Special thanks to the authors and researchers for their comprehensive contribution to this growing field.



Comments
Post a Comment